Psoriasis Digital Health Challenge | 武田薬品

Psoriasis Digital Health Challenge
日本語版はこちら
OVERVIEW
Digital Health Corporate Partnership Program in Psoriasis
Takeda is calling for ideas from organizations with digital technology to solve challenges faced by psoriasis patients, their families, and healthcare professionals, in collaboration with Plug and Play Japan, Inc. Takeda is soliciting companies with digital health solutions aimed at improving the Quality of Life (QoL) of psoriasis patients and their families and supporting physicians in their patient care.
With over 30 years of experience in gastroenterology and a strategy to expand on our core expertise in inflammation, Takeda is developing treatments in areas of high unmet need including inflammatory bowel disease, celiac disease, psoriasis, advanced liver disease, and neurogastric disorders. In recent years, Takeda has expanded our experience and expertise to address the unmet needs in psoriasis, a disease characterized by immune system abnormalities that cause inflammation of the skin.
Many patients with psoriasis face not only physical pain and psychological burdens from the disease, but also various social challenges1. Takeda aims to contribute to improving the environment surrounding patients living with psoriasis.
Program Timeline
- Application Period: September 29, 2025 - November 18, 2025
- Selection Period: November - December 2025
- Acceleration Program: January - March 2026
- Demo Day (Final Presentation to Takeda): March 2026
Program Benefits
- Opportunity to jointly conduct a Proof of Value (PoV) with Takeda
- Mentoring and guidance from Takeda experts
- Opportunity to pitch solution ideas to Takeda's senior leaders
- Potential for further collaboration with Takeda
- Networking and engagement opportunity with Takeda and Plug and Play Japan*
* Takeda’s partner for the Psoriasis Digital Health Challenge program(https://japan.plugandplaytechcenter.com/)
PROGRAM
PROGRAM DETAILS
- In the Psoriasis Digital Health Challenge, Takeda has identified the following five key challenges and is soliciting cutting-edge technology and solution ideas to address them.
- Promoting Communication in Psoriasis Care
- Enhancing Medical Collaboration and Reducing the Burden of Screening Tests in Treatment Intensification
- Supporting psoriasis patients in maintaining treatment and daily life
- Early consultation and diagnosis for psoriasis
- Eliminating prejudice stemming from insufficient understanding of the disease and the social and psychological isolation of patients
- Selected participants will receive tailored mentoring and support throughout a two-month acceleration phase to refine their solution ideas and conduct Proof of Value (PoV).
- At the final “Demo Day” event, participants will present their collaboration proposals to Takeda’s senior decision-makers. We aim to explore opportunities for long-term collaboration with participants. Join us in co-creating value in healthcare—helping to bring hope and solutions to patients and their loved ones worldwide.
THEME DETAILS
1. Promoting Communication in Psoriasis Care

Background:
- In psoriasis care, limited interview time and differences in disease understanding between doctors and patients make it difficult to share treatment goals, leading to challenges in making satisfactory treatment choices and continuing treatment4,5. Treatment may be progressing without meeting the patient's needs due to the doctor's lack of awareness and communication to patients about the risk of psoriatic arthritis, systemic diseases, and comorbidities, and the inability of patients themselves to communicate their problems to the doctor4,5.
- The time required to feel the effects of treatment and the unclear evaluation criteria and timing also lead to patient anxiety and a decline in motivation for treatment5,6. Due to these circumstances, it has become difficult to practice SDM (Shared Decision Making)* and provide treatments that consider QoL.
- Therefore, there is a need for flexible and effective solutions that enable information provision to support the construction of mutual understanding and trust between doctors and patients, and to create an environment where patients themselves can participate in treatment while understanding and convincing.
* SDM(Shared Decision Making):The process by which patients and medical persons make medical decisions together.
2. Enhancing Medical Collaboration and Reducing the Burden of Screening Tests in Treatment Intensification
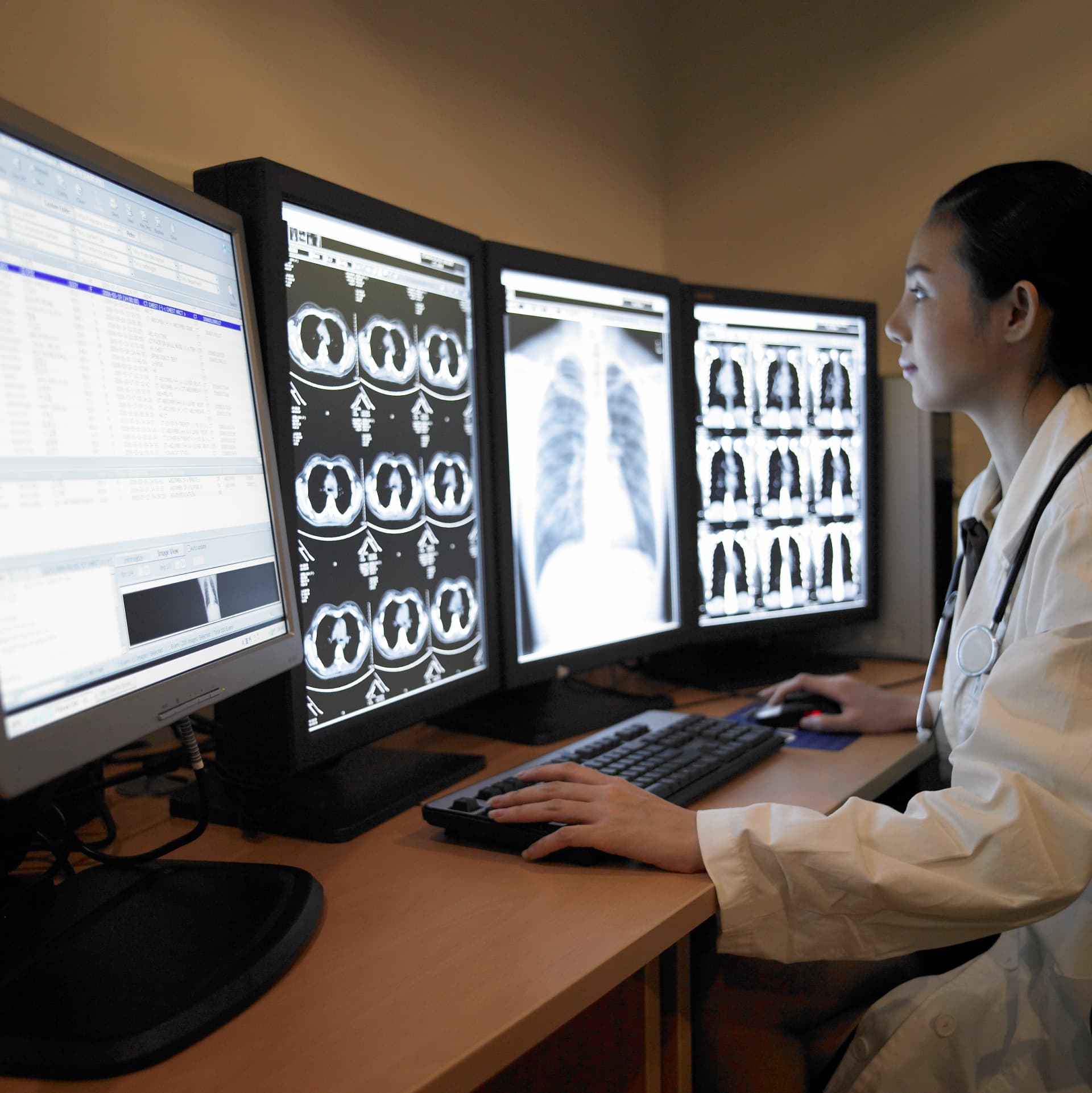
Background:
- While some treatment options are great hope for psoriasis patients, there are multiple barriers to implementation.
- For example, treatment options that require pre-testing for TB risk assessment (e.g., chest X-rays and blood tests) may require collaboration with other healthcare providers in clinics that do not have testing equipment.
- Some treatment options may vary in adoption depending on the facility’s approval status and the physician’s treatment policy.
- There is a need for solutions that create an environment where patients who need therapeutic drugs can access them smoothly.
3. Supporting psoriasis patients in maintaining treatment and daily life

Background:
- Psoriasis treatment is often long-term, leaving patients with anxiety and burden not only at the beginning of treatment, but also at all stages of treatment selection, continuation, and interruption.
- In particular, the correct understanding of the disease and the treatment chosen and concerns about the financial burden are challenges in continuing appropriate treatment4,7.
- Additionally, physicians also have limited consultation time, and they may find it difficult to provide support for issues affecting patients’ daily lives9.
- Under these circumstances, solutions are needed to stay close to patients' lives while maintaining motivation for treatment and improving adherence.
4. Early consultation and diagnosis for psoriasis

Background:
- Lack of disease knowledge on the part of patients may be the cause of delaying medical visits and access to appropriate medical care, which may be due to the neglect of initial symptoms such as appearance and itching2.
- Even doctors may find it difficult to distinguish psoriasis from other skin diseases (such as seborrheic dermatitis or eczema), which often delays proper diagnosis or leads to misdiagnosis3.
- As a result, patients may take time to start appropriate treatment, so solutions are needed to solve these initial delays and diagnostic accuracy challenges.
5. Eliminating prejudice stemming from insufficient understanding of the disease and the social and psychological isolation of patients

Background:
- Psoriasis has visible symptoms and is considered to be a disease that is susceptible to stigma and misunderstanding in social life due to its appearance4,8,9.
- Patients are exposed to prejudice and misconceptions such as "unhygienic diseases" and "infectious diseases" in everyday situations such as work, school, and home, and tend to hide their symptoms more than necessary. As a result, loneliness and psychological stress such as "no one can sympathize with or understand me" and "I don't have friends with the same disease" intensify, which is thought to have a serious impact on their QoL and mental health4,8,9.
- In addition to eliminating this social and psychological isolation and promoting correct understanding of the disease to the world, solutions are needed to create systems that allow patients to connect with society with peace of mind, such as empathetic experiences and support.
ELIGIBILITY
- Applicants must be organizations (not individuals) with concepts, prototypes, or products related to the program themes and must be able to conduct Proof of Concept (PoC) activities in Japan.
- Applicants must be able to communicate in either English or Japanese.
BENEFITS
Participants of this acceleration program will receive the following benefits through this program:
- Opportunity to conduct a joint Proof of Value (PoV) with Takeda: Takeda will support participants in co-conducting a PoV tailored to specific challenges related to the program themes.
- Access to mentoring from Takeda experts: Participants will receive guidance and feedback from Takeda’s subject matter experts across relevant functional areas.
- Pitch opportunity to Takeda leadership: Participants will be invited to present their validated concepts and collaboration proposals directly to Takeda’s senior management team.
- Opportunity to explore future collaboration with Takeda: Based on the outcomes of the PoV, Takeda may consider potential long-term partnerships such as joint development, licensing, or manufacturing agreements. However, future collaboration is not guaranteed.
- Networking and engagement with Takeda and Plug and Play: The program includes networking events and workshops with healthcare experts from Takeda and Plug and Play to foster meaningful connections.
ENTRY
Applications are now closed. Thank you for your interest.
Application Deadline:November 18, 2025, at 23:59 JST
The orientation session has ended. We appreciate your participation.
Date & Time:October 24, 2025, 13:15-14:15 JST Location: Takeda Tokyo Global Headquarters & Online Registration Deadline:October 21, 2025, at 15:00 JST(Online attendance registrations close on October 23, 2025, at 23:59 JST)
IMPORTANT NOTICE
- Please do NOT submit any confidential information at the time of application
- After the selection period a participation agreement will be signed between the selected organizations and Takeda to protect intellectual property. In addition, a non-disclosure agreement (NDA) will be executed among each selected organization, Plug and Play Japan, and Takeda.
- This program is NOT intended to promote or advertise any pharmaceutical product under development or on the market, and is NOT an investment solicitation
Q. How much time is required to participate? A. Required events will take place during the two-month program period. Additional collaboration will be based on mutual agreement.
Q. How many organizations will be selected to enroll in the program? A. Up to three participants are expected to be selected.
Q. Will participants receive funding or investment? A. Not guaranteed. However, depending on “Demo Day” outcomes, potential investment may be considered.
Q. Will the program be held online or in person? A. The program will be primarily online, with select in-person events.
Q. Are there any restrictions on the technology readiness level of solutions? A. No. Solutions at all levels of technological maturity are welcome, including early-stage ideas and prototypes.
- Siva Narayanan, Victoria Guyatt, Alessandra Franceschetti, Emily LHautamaki: Disease burden and patient reported outcomes among patients with moderate to severe psoriasis: an ethnography study: Psoriasis: Targets and Therapy (2014)
- Azura Mohd Affandi, K. Thiruchelvam. Patient perspective on psoriasis: Psychosocial burden of psoriasis and its management in Malaysia, PLOS ONE, 2024
- Suzanne Murray, Jeffrey Crowley, Melinda J. Gooderham, et al. Healthcare Providers Face Numerous Challenges in Treating Patients with Psoriasis: Results from a Mixed-Methods Study. Journal of Psoriasis and Psoriatic Arthritis, 2022, vol.7(1) 25-43
- Japan Psoriasis Association. Global report on PSORIASIS 2016. Accessed on September 1, 2025. https://iris.who.int/bitstream/handle/10665/204417/9789241565189-jpn.pdf
- Parker J Magin, Jon Adams, Gaynor S Heading and C Dimity Pond. Patients with skin disease and their relationships with their doctors: a qualitative study of patients with acne, psoriasis and eczema. The Medical Journal of Australia, 2009; (2): 62-64
- Katherine K. Brown, Wingfield E. Rehmus. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. Journal of American Academic Dermatology. 2006
- Tina Bhutani, Jillian W. Wong. Access to Health Care in Patients With Psoriasis and Psoriatic Arthritis. JAMA Dermatol Published Online; 2023;149 (6): 717-721
- Japan Psoriasis Association. A Guide for Working Psoriasis Patients (Hataraku kansen kanja-san no tame no gaido). Accessed on September 1, 2025. http://www.jpa1029.com/archives/hataraku2024.pdf
- IFPA. Inside Psoriatic Disease FAMILY. Accessed on September 1, 2025. http://jpa1029.com/files/Inside-Psoriatic-Disease-Family-Report_JPN2.pdf
CONTACT
For inquiries regarding this program, please contact us at the following email address. Takeda Pharmaceuticals Psoriasis Digital Health Challenge Office: [email protected]
